45 fda approved health claims on food labels
Pet Food Labels - General | FDA Pet food labeling is regulated at two levels. The federal regulations, enforced by the United States Food and Drug Administration (FDA), establish standards applicable for all animal feeds: proper ... Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ...
FDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20(b)(2), if scientific information on the human food safety aspect of the use of the approved human drug in food-producing animals is not available, the veterinarian ...

Fda approved health claims on food labels
Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003. Is It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
Fda approved health claims on food labels. Pet Food | FDA - U.S. Food and Drug Administration For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ... Is It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA U.S. Food and Drug Administration, Guidance for Industry, Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements July 2003. Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...








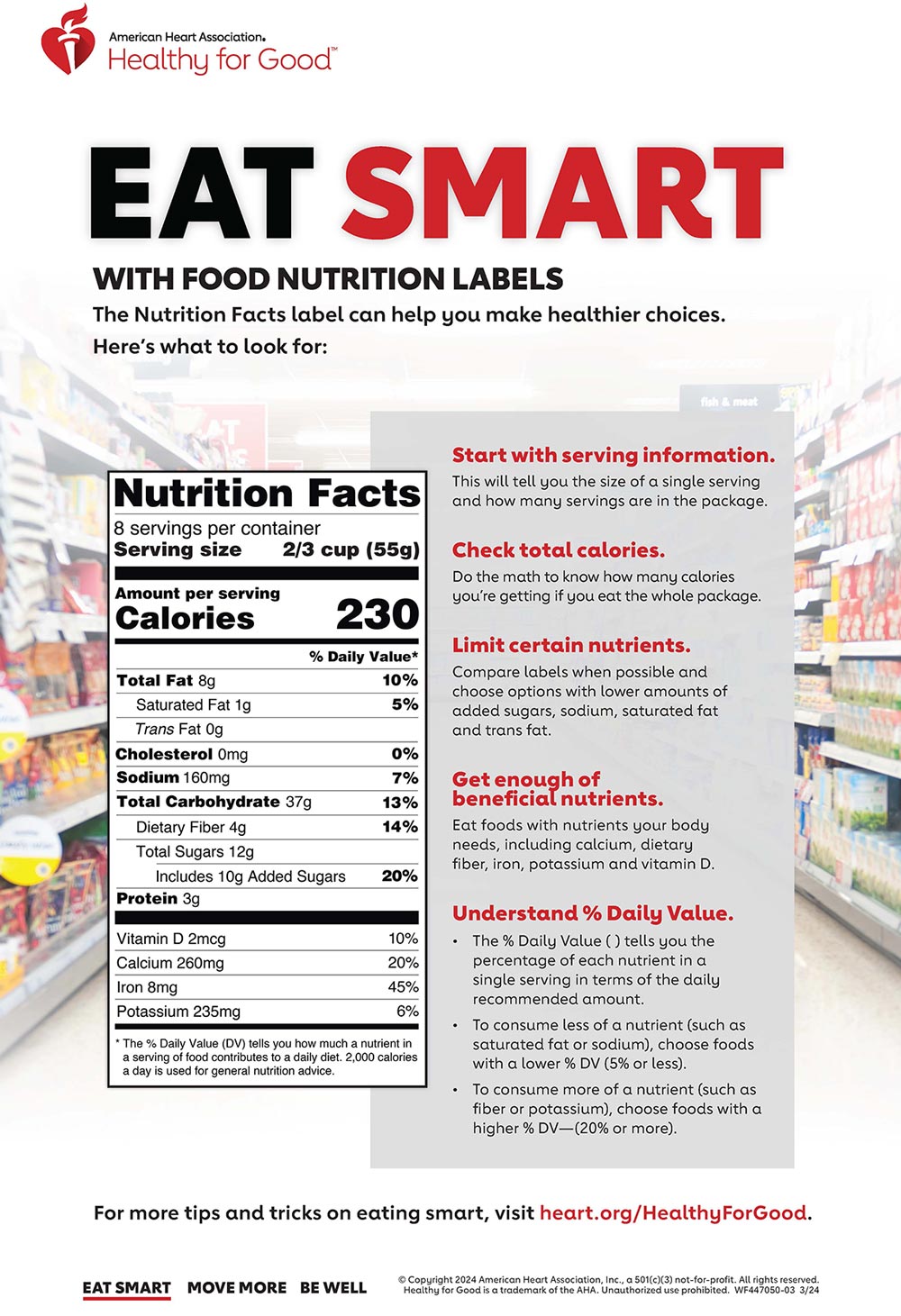






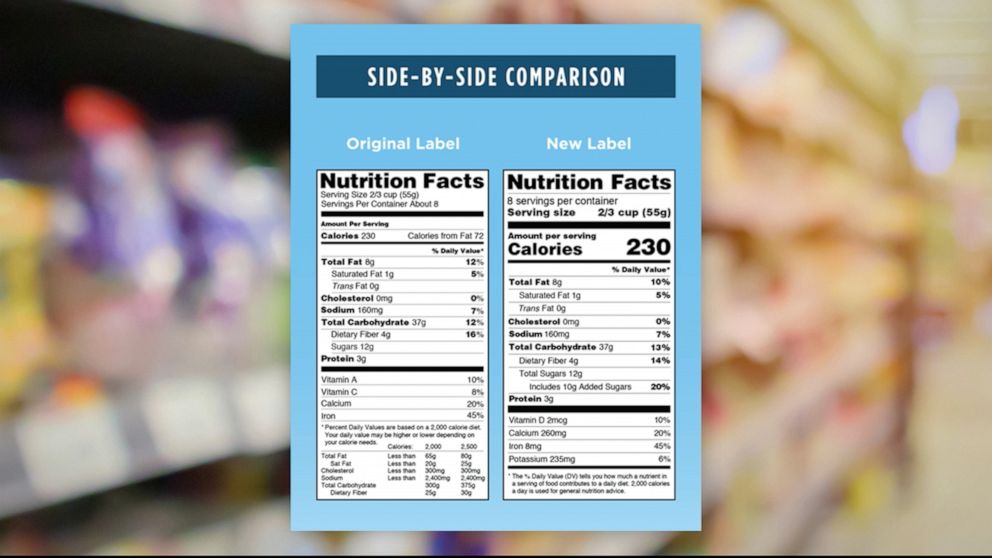







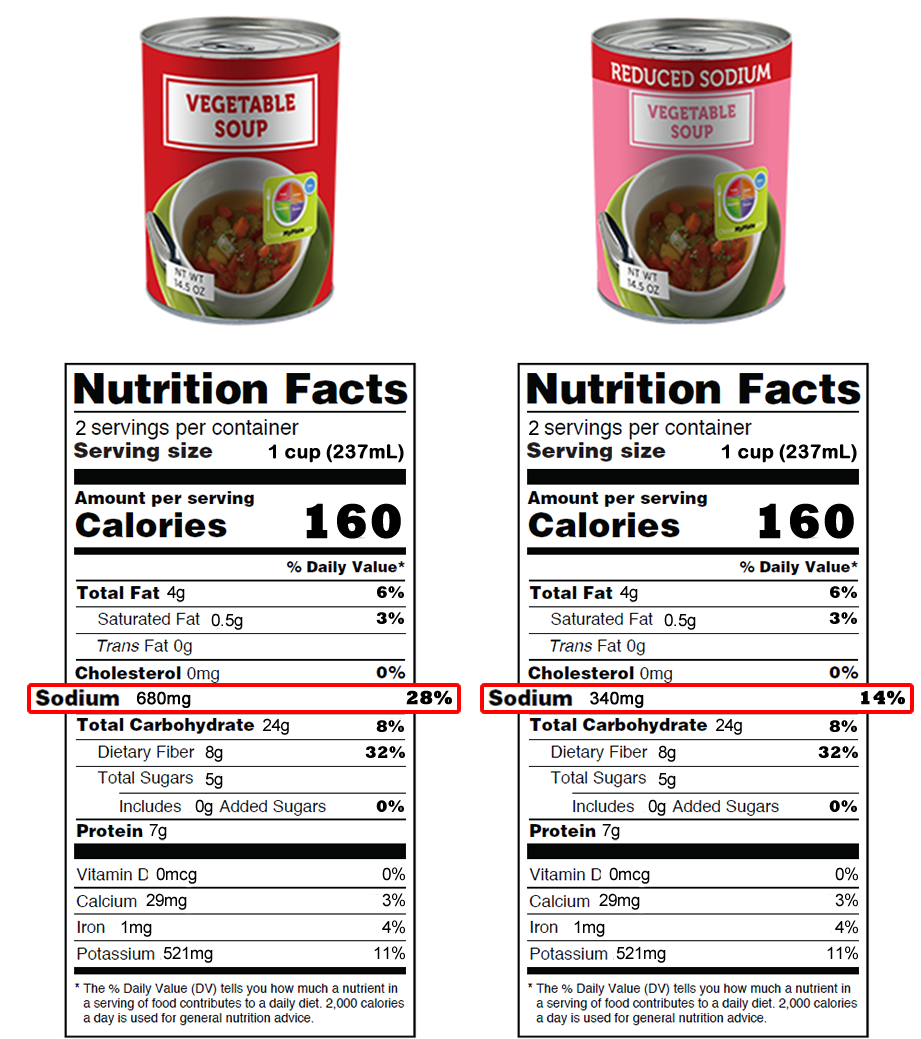












Post a Comment for "45 fda approved health claims on food labels"